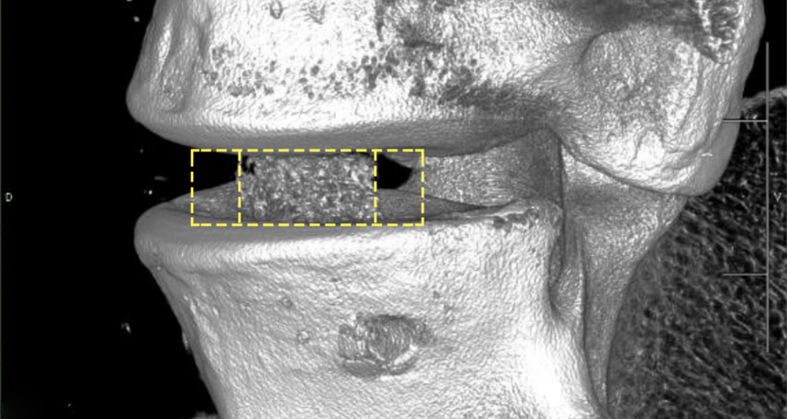
Bond3D has partnered with Invibio Biomaterial Solutions to enable the development of the fourth generation of PEEK-based spinal cages.
Bond3D’s capabilities enable medical device companies to design and print PEEK spinal cages, and the company has matured its technology to print high-strength PEEK parts with properties comparable to PEEK components that are injection molded or machined. The company believes that its technology will catalyze the development of further solutions in medical devices by allowing the creation of high-strength, porous implants to permit bone ingrowth and increase device fixation and also allow the rapid printing of patient-specific implants.
The company’s cages are said to have sufficient biocompatibility to gain FDA clearance. Currently, the final steps toward FDA submission are being taken with a US-based spine company, supported by a masterfile that will facilitate subsequent medical OEMs to bring different designs to market.
In support of adoption in the spine market, Bond3D and Invibio seek to:
- Prove voidlessness and high strength in PEEK parts in elementary directions
- Test highly porous PEEK cages to ASTM F2027
- Produce controlled regular and complex porous structures within spinal cages
- Ensure the reproducibility and robustness of the printers, the supply chain, documentation and traceability
Source: Bond3D
JAV
Julie A. Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.




