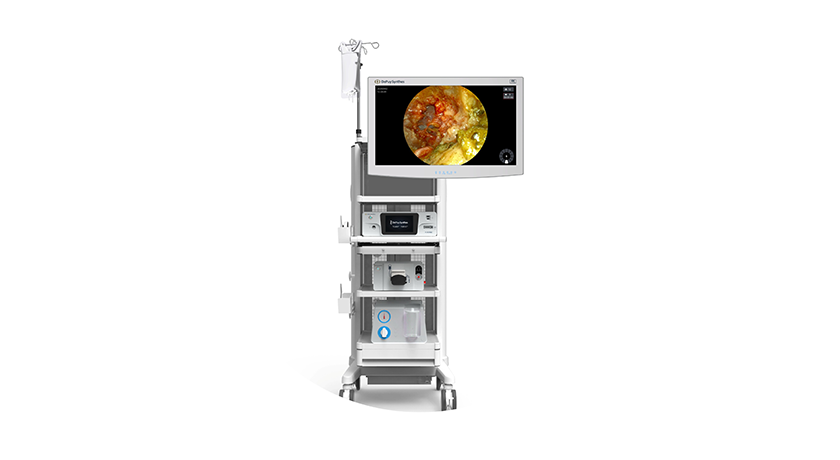
DePuy Synthes was granted FDA 510(k) clearance to market the TELIGEN™ System, an integrated technology platform that supports minimally invasive surgical transforaminal lumbar interbody fusion (MIS-TLIF) procedures through digital tools for visualization and access.
TELIGEN will be available in the U.S. later this year.
TELIGEN comprises a tower that delivers a suite of technologies, including camera control, a VueLIF-T™ Procedure Kit with disposable HD camera, a TELIGEN Clear Discectomy Device and patient-based disposable ports. TELIGEN integrates with the UNLEASH™ bundle of implants, which is designed to streamline the main stages in MIS-TLIF.
The digitally enabled TELIGEN VUE Camera, located at the distal end of the patient-specific port, is designed to eliminate the need for a microscope and can provide an unobstructed view of the surgical site. It offers hands-free visualization during the procedure, along with a multidirectional and expanded field of view. Additionally, the self-cleaning camera includes LED lighting and allows surgeons to manipulate image clarity based on their preference. A heads-up display allows surgeons to maintain ergonomic posture during procedures.
Based on a small cadaveric study, TELIGEN can reduce fluoroscopy time by 47% compared to MIS-TLIF procedures performed using a surgical microscope. It also provides a reduction in instrument trays and processing costs per surgery for the 10 additional trays not required with the TELIGEN™ System.
Source: DePuy Synthes
JAV
Julie A. Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.