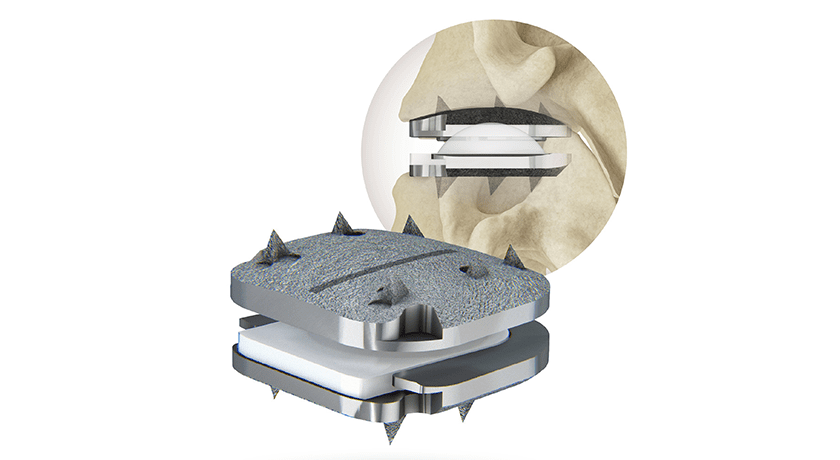
Centinel Spine announced the first implantation of its prodisc® C Vivo Cervical Total Disc Replacement (TDR) device. In July, the company received FDA approval for 1-level indications for prodisc C Vivo, prodisc C SK and prodisc C Nova.
The prodisc C Vivo has been in clinical use internationally since 2009 and is reported to be one of the most frequently implanted TDR devices outside of the U.S. The device has keel-less fixation and combines an anatomically-designed superior endplate with lateral spikes to optimize fit and provide immediate fixation.
Similar to all prodisc products, the prodisc C Vivo device incorporates prodisc CORE technology, the basis behind the predictable clinical outcomes of the prodisc platform after 30 years and over 225,000 implantations worldwide.
Source: Centinel Spine, LLC
JAV
Julie A. Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.




