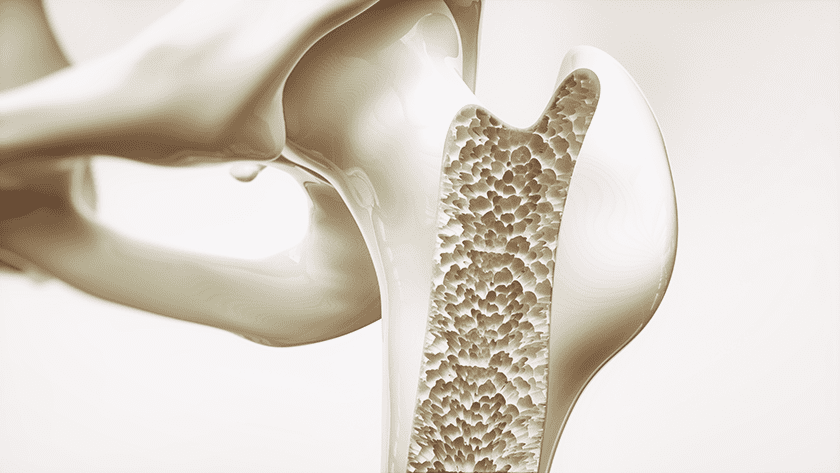
CurveBeam AI’s medical diagnostic software, OssView™, received FDA Breakthrough Device Designation. OssView calculates a Structural Fragility Score (SFS) which determines bone microstructural deterioration, a clinical aid to assist a medical provider in determining bone fragility and fracture risk in over 70-year-old females.
Bone mineral density (BMD) measurement, obtained via dual energy x-ray, is the current standard of care to determine fragility fracture risk in patients. In some cases, other fracture risk software tools are used to further identify fracture risk. When compared to these techniques, SFS has shown performance to support a Breakthrough Device Designation.
Bone density defined-osteoporosis can miss up to 80% of fragility fractures. BMD measures the amount of bone but not the breakdown of three-dimensional bone architecture.
SFS is calculated from a high resolution peripheral quantitative computed tomography scan of the wrist. CurveBeam AI intends to offer point-of-care, high resolution CT platforms to improve access to patients for medical providers dealing with patients who are non-osteoporotic.
Earlier this month, CurveBeam and StraxCorp entered into a definitive merger agreement to form CurveBeam AI. The new entity AI expands CurveBeam’s point-of-care imaging solutions into the bone health space, as well as artificial intelligence (AI)-driven applications for weight-bearing CT imaging.
“We are extremely pleased to be moving forward with the SFS diagnostic through the FDA Breakthrough Device Program,” said Greg Brown, CEO of CurveBeam AI. “There is a clear need for an improved clinical aid like SFS to help clinicians more effectively assess bone health and prevent fractures. We are looking forward to progressing its clearance with FDA.”
Source: CurveBeam AI
JAV
Julie A. Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.




