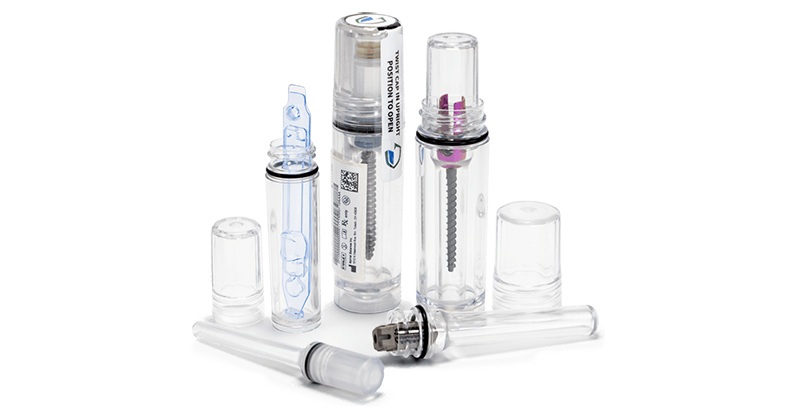
Guardian Medical has successfully completed 10-year accelerated aging testing for their pre-validated CapSure™ sterile barrier packaging system. The company designs, manufactures and sells CapSure, a tube packaging system for medical devices in fields such as orthopedics, craniomaxillofacial and dental.
“Four years ago, when we started Guardian Medical USA, we were determined to introduce an innovative package, incorporating a unique seal capable of setting a new standard in sterile barrier packaging. Earlier this year we completed 7-year shelf-life testing and were thrilled with the results, but our dedicated team was confident we could achieve more,” said Guardian Medical USA President and CEO Tracy Momany. “Our clients are looking for packaging solutions that can reliably pass validations and thereafter provide lasting performance along with tangible economic and environmental benefits. Just look at a CapSure package and you understand the value of having an economy of size, where less is better! And that same package is fully recyclable.”
“At a time when transportation and supply costs are increasing and there are shortages of certain packaging supplies, being able to step up and deliver a pre-validated sterile barrier package system having a 10-year shelf life is extremely important. Our industry has been built on constant improvements and advancements in technology, materials, design and procedural instrumentation with the end goal begin a better outcome for the patient and surgeon. Guardian Medical has taken the same approach and delivered a packaging system that embraces and embodies those same tenants,” said Don Kennedy, VP Sales and Marketing.
Source: Guardian Medical USA
JAV
Julie A. Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.




