
Nature is the name of the game when it comes to an ideal bone regeneration scaffold. Thus far, synthetics and allografts have dominated the orthopedic implant market despite their known drawbacks. But Zoan BioMed, a Galway, Ireland-based medtech company, is taking a different route by developing a bone scaffold sustainably sourced from marine coral.
The Technology
Throughout their R&D work, Zoan BioMed observed the inherent structure and porosity of the scaffolds of coral and the similarities it carries to human cancellous bone. The concept that coral is a highly effective bone graft substitute is not new, but previous attempts to use it have not been successful.
“There are thousands of different species of coral in the world; they’re all different and have different characteristics,” said Stephen Wann, CEO of Zoan BioMed. “A big part of our R&D program was identifying a small number of those species of corals that were a very close mimic to human cancellous bone. That was the starting point.”
Zoan BioMed partnered with the National University of Ireland, Galway (NUIG) to study how the coral scaffolds interacted with human cells in the lab. Dr. Cynthia Coleman, Assistant Professor in Cellular Manufacturing and Therapy at NUIG Regenerative Medicine Institute, observed that the interaction between the scaffolds and human cells was quite extraordinary in that not only did the cells attach to the coral scaffold in an encouraging way, but they actually proliferated.


Zoan BioMed is using coral granules to make bone graft substitutes.
The observations acted as a launchpad for developing a revolutionary bone graft material.
A Sustainable, Repeatable Approach
A major orthopedic company previously attempted to develop a coral-based bone graft that didn’t pan out. The type of coral chosen didn’t have enough inherent strength, and it resorbed too quickly to be effective, Wann said. Zoan BioMed has taken a different approach.
“There are many species of coral, but also within that, if you’re taking coral from the wild to do something like this, every individual colony is different,” said Martin Johnson, Head of Product R&D at Zoan BioMed. “They’re genetically different; they have different porosity, different strengths, and different growth patterns.”
Zoan BioMed found a solution to that challenge by mastering the ability to cultivate coral in an indoor facility. They built manufacturing capability themselves, giving the company a unique, inherent understanding of the material and how it grows, how it can be cultivated and produced, and how it interacts with human cells.
After a one-time event of licensed extraction of small samples of several species of coral from the wild, they’ve been able to reproduce different types of coral at scale, many times faster than they would grow in the wild.
“We have never been back to the ocean to replenish our stock, nor will we ever go back to the ocean to replenish that stock,” Wann said. “We have a uniquely sustainable source of our material through a great feat of engineering.”
“We’ve developed the know-how and the patent-pending technology to be able to propagate clonally-identical coral at speed,” Johnson said. “We can grow our biomaterials cleanly, we can grow it quickly and without energy- or materials-intensive steps.”
Every batch of coral used in Zoan BioMed’s products is chemically and physically the same. Johnson and Wann consider this reproducibility in a biomaterial as one of the platform’s greatest strengths. Furthermore, Zoan has control on the biomaterial properties.
“Through controlling the growth conditions, it is possible to modify the chemical and physical properties of coral, allowing us to target different potential applications in orthopedics and next-generation biomaterials development,” Johnson said.
Zoan is developing a uniquely environmentally sustainable orthopedics platform and seeks to become the first carbon neutral bone graft.
“In addition to never returning to the ocean to harvest coral, we are working toward being the first carbon neutral bone graft, accounting for supply chain, production and distribution,” Wann said. “Very few people in the medical device world are thinking about sustainability right now. We are well ahead of the curve on this and we are confident it will become increasingly important in the coming years.”
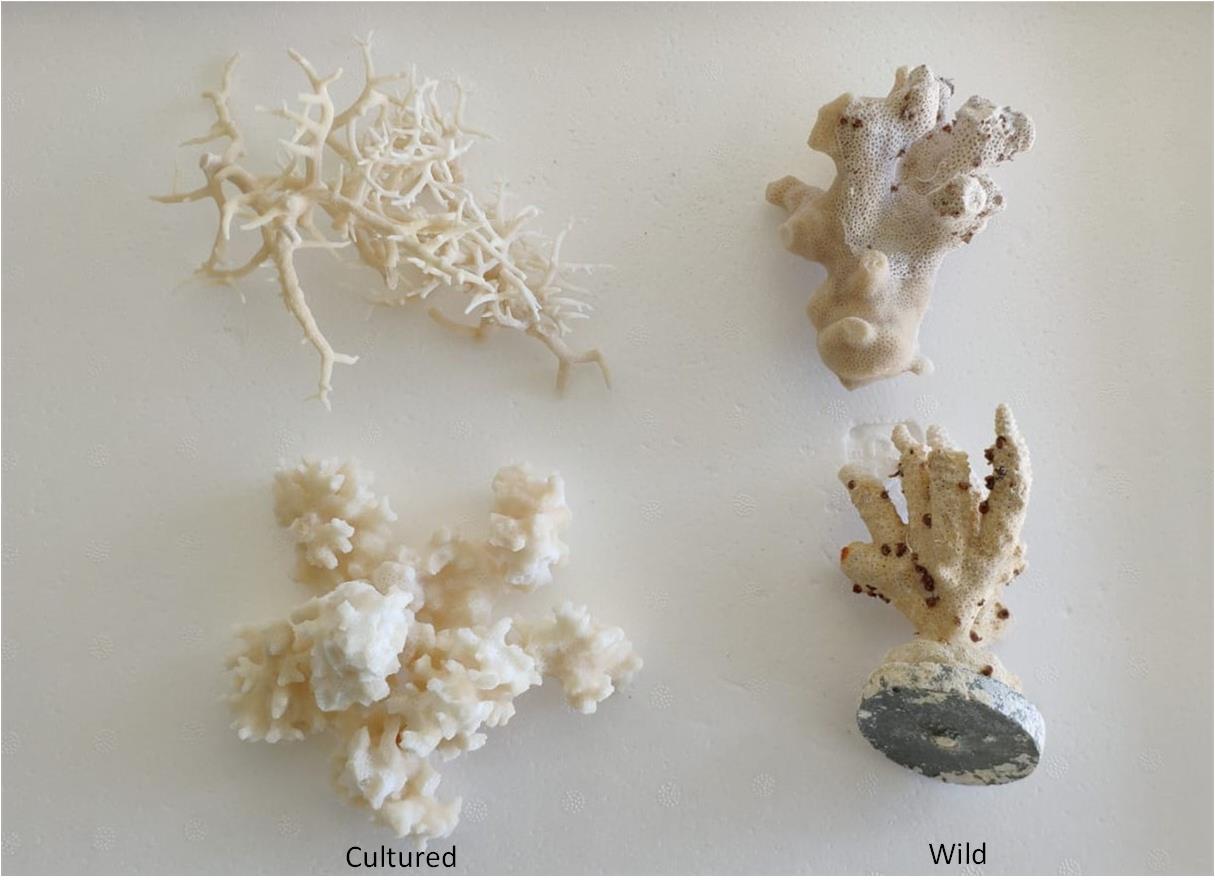

The various species of coral are genetically different, with different porosity, strengths and growth patterns. Zoan BioMed found a solution to that challenge by mastering the ability to cultivate coral in an indoor facility.
How it Works
Coral’s strength and porosity are key to its success as a bone graft substitute. The porosity of the material itself and the porosity of the granular biomaterial that it produces make it well suited for vascularization. Combine that with its biocompatibility, and the results have been promising.
“Sea water naturally contains strontium, magnesium and zinc and various other metal ions that are beneficial for bone formation,” Johnson said. “The corals lay these down into a porous scaffold composed of crystals of aragonite, mainly, which is a calcium carbonate mineral along with some inclusions of other ions.”
That makes for a very welcoming environment for bones. In animal trials, Zoan BioMed witnessed bone cells moving into the coral and growing on it. The coral particles act as “piers” for the trabecular bridging across the defect, providing an important structural component to bone formation.
“In our animal trial, we see zero adverse host reaction,” Johnson said. “There is no piece of coral in any of the animals that we implanted that is not completely surrounded by and in contact with new bone growth within about 13 weeks. The pathologist who was analyzing the histology from the animal trial was astonished by this behavior and said that bone ingrowth was as dense as it could possibly be. It was really optimal healing. Ultimately, we believe that the coral, when it’s advanced in humans, will give the best patient outcomes.”
Looking Ahead
Zoan BioMed’s launch product is a granule for trauma and extremity applications, currently with FDA for 510(k) clearance. The expectation is that there will be broad use across orthopedics as a base material for composites. The strategy is to get proof of concept through the granule to prove out the core capabilities and mechanics of the material before actively and aggressively going after the spinal market.
Once FDA clearance is given, Zoan BioMed expects to immediately move to human trials. Several surgeons in the U.S. and Europe have expressed interest in working with Zoan BioMed to have the first-in-human trial.
Recently, Zoan BioMed and CELLINK, a world leader in bioprinting, signed a partnership agreement and began working together to develop the material in a 3D-printed format. The vision is to create bioprinted personalized bone grafts as well as bone tissue models and scaffolds for R&D and device testing.
Leveraging R&D funds to build partnerships in academia and with other commercial operations to develop the next generation of coral biomaterials is key to Zoan’s future potential, according to Johnson.
HT
Heather Tunstall is a BONEZONE Contributor.




