
Stacked up against other orthopedic implants, shoulder replacements pose unique challenges to device makers due to their complexity. But in 2022, ever-evolving technologies are giving companies the power to overcome them and improve patients’ lives in ways that weren’t possible ten or even five years ago. BONEZONE talked with experts in this growing orthopedic market to learn what’s driving growth in the sector, and how burgeoning technologies will likely shape shoulder replacement implants over the coming years.
Shoulder Replacement Market Drivers
Rajit Kamal serves as Worldwide President of Sports Medicine/Shoulder Reconstruction at DePuy Synthes. Citing a global market that he estimated is growing at 7% per year, Kamal credited demographics and transformative new technologies for the shoulder replacement market’s expansion.
“There are a couple of things I think that are driving growth. One is demographics. One indication of total shoulder arthroplasty is a post-arthritic joint. About 40 million people globally are symptomatic in that way,” he said, adding that as populations grow globally, that number will increase.
“The second indication is rotator cuff tears,” Kamal said. “We estimate about 60 to 70 million people have rotator cuff tears, and about 30% of those are classified as irreparable. I think that’s another tailwind in terms of volume. There are more structural drivers as well.”
Kamal acknowledged that shoulder replacement isn’t as mature as its counterparts in hip and knee. He remarked that new research, data, evidence and surgeon specialization are spurring its development alongside boosts from burgeoning technologies and new markets opened up in locations such as southeast Asia. When asked how the market will evolve, Kamal portrayed an industry indelibly impacted by COVID-19 through an accelerated shift towards ASCs and a newfound adoption of technology.
“Five years back, when I spoke with surgeons, I would say maybe 1% of my conversations were about technology, and today it’s 80%. So, it has completely shifted, and that shift has happened drastically during COVID,” Kamal said. “There’s something called ‘crossing the chasm’ technology adoption. COVID pushed the industry through that chasm.”
Kamal noted that new technology solutions are helping to make surgeries easier for surgeons and improve patient outcomes. The burgeoning technological developments are related to the needs and perceptions of orthopedic surgeons.
“More and more, I’m hearing, ‘Give me solutions that are more efficient in the O.R. that require less storage space.’ Surgeons are asking for products and technologies that have fewer trays and fewer imperative steps that are also more efficient from an inventory standpoint,” Kamal said. “The second thing is technology solutions. More surgeons want surgical planning because it has benefits in terms of managing inventory.”
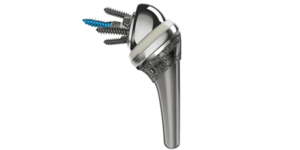
The INHANCE™ Shoulder System from DePuy Synthes received FDA 510(k) clearance for use in reverse total shoulder arthroplasty in May. This clearance is in addition to the system’s already cleared use in anatomic shoulder arthroplasty. Photo courtesy of DePuy Synthes.
Kamal pointed to imaging technologies as essential tools that are helping orthopedic surgeons make critical decisions in the O.R. before surgery. For Kamal, being at the forefront of the technological transformation within the shoulder replacement market is exciting.
“You’ll see significant adoption of technology that ensures a much higher value proposition. Being part of that, being able to shape that, is exciting,” Kamal said.
Streamlining Implants and Instruments
David Blue is the Chief Commercial Officer for Shoulder Innovations, a Michigan-headquartered orthopedic device company focused on developing innovative shoulder replacement technology.
“We love being change agents in this innovative space,” Blue said, “and focusing on solving the real problems that need to be addressed.”
Blue remarked that his company has gained significant acclaim and momentum due to its initial invention of the InSet technology, a stabilization glenoid system that addresses the “rocking horse effect” in shoulder implant recipients. The rocking horse effect occurs when the humerus both rotates and translates on the scapula as it articulates.
“Because we developed a solid solution for addressing glenoid loosening, we’ve taken that product and expanded it even further. We have so much innovative design embedded into that glenoid. It has straightforward instrumentation that makes it special, including not having to worry about radius of curvature mismatch,” Blue said. “We used to have to worry about what size glenoid went with what size humeral head. Now, it doesn’t matter what size head you use with what size glenoid. You can now put the largest head on the smallest glenoid or the smallest head on the largest glenoid. This creates unique flexibility that is recognized and appreciated by surgeons.”
Blue highlighted another significant Shoulder Innovations benefit for surgeons: consolidation and streamlining of implants and accompanying instruments. Rather than requiring large quantities of complex instruments and implants to perform the shoulder replacement surgery, surgeons and hospital staff who work with Shoulder Innovations can expect the same widespread offering and results from only one neatly organized container.

In late 2019, Shoulder Innovations was granted FDA 510(k) clearance to market InSet Plus™ augmented glenoids for the InSet™ Total Shoulder (pictured). Photo courtesy of Shoulder Innovations.
“Being able to build a complete shoulder system from the ground up has allowed us to provide everything into a true and completely integrated system,” he said. “We call it ‘the value and power of one,’ where you can provide one set of instruments that can work with all our various product options. it’s pretty amazing, because the cost of sterilization labor, wrapping and prepping of the instrument trays and getting them ready for preoperative surgery is about $150 to $250 per tray, depending on where you are in the country. If other companies bring in five or six trays compared to our one tray, we’re providing a huge cost saving to the healthcare facility.”
Enabling and Digital Technology Evolution
Tim Lanier serves as Vice President & General Manager of the Upper Extremities group for Stryker. Having described his company as going “all in on digital,” Lanier predicted that technology will help continue to make surgeries quicker and easier for patients without sacrificing patient outcomes.
“We’re embarking on a new technological platform around customizing a patient’s implant blueprint through our digital software system,” he said. “Surgeons will now be able to reduce some of the steps required to do the case. The one big one is the amount of bone they take out during the reaming step.”
Lanier added that technology helps errors to be eliminated by removing some of the technical steps for surgeons. Additionally, patient outcomes are improved in the process. He highlighted personalized medicine as beneficial to hospitals due to the reduced amount of instrumentation and implants needed for surgeries, a change he predicted would translate to lower costs.
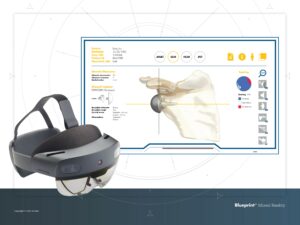
Stryker executives said the company is going “all in on digital” and is leveraging its Blueprint technology to make surgeries quicker and easier for patients without sacrificing patient outcomes. Photo courtesy of Stryker.
“Another big trend is going to be robotic technology — everybody will be asking for it,” Lanier said. “The surgeons who do five to 100 cases a year are the ones who will benefit from enabling technologies like navigation or robotics. They’re going to be designed to make [surgeons] better. They’ll be able to bill more cases because some of these enabling technologies will enhance the operative process.”
After remarking that Stryker will not “shy away” from the industry trend toward ASCs, Lanier shared his thoughts on how digital technology has touched and developed every aspect of the company he serves.
“We have built our business around what we would call a digitally enabled hardware model,” he said. “The digital platform Blueprint is a conduit to everything we do, whether it’s pre-planning, how we think about manufacturing and optimizing implant design, like patient-matched implants, to how we think about reducing cost through our supply chain process and training sales reps. It touches everything that we do.”
PM
Patrick McGuire is a BONEZONE Contributor.




