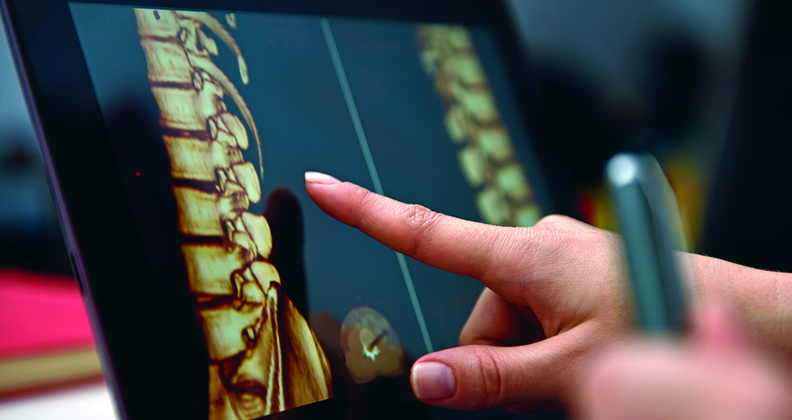
Medtronic received FDA 510(k) clearance for its UNiD Spine Analyzer v4.0 planning platform, which includes a new Degen Algorithm for degenerative spine procedures. The algorithm leverages machine learning to help surgeons plan and personalize procedures for patients undergoing lower lumbar spine surgery and predicts spinal compensation mechanisms six months after the operation.
The new update also includes enhancements to the pediatric and adult deformity algorithms predicting compensatory changes to the spine. Medtronic is reportedly the first and only company to have FDA cleared predictive models for spine surgery.
The release comes with a new UNiD™ Hub patient-centric platform that enables surgeons to track patients throughout the perioperative care pathway and assess surgical results through long-term radiographic and patient-reported outcomes data collection.
“Patient by patient, our UNiD Lab engineers have learned from more than 10,000 spine surgery cases to deliver greater insights to surgeons that lead to better patient alignment,” said Dan Wolf, Vice President and General Manager, intelligent Data Solutions within the Cranial & Spinal Technology business, part of the Neuroscience Portfolio at Medtronic. “It is truly exciting to share that we have expanded our UNiD ASI technology to include hardware and software solutions dedicated to helping spine surgeons treat degenerative spinal pathologies, where the majority of spine surgery is performed.”
Over time, UNiD ASI technology will continue to evolve as more case data is added and predictive algorithms are further refined to help make spine surgery more predictable for surgeons and patients alike.




