
Evonik’s recombinant, non-animal-derived collagen platform, Vecollan, is now available at commercial scale. Vecollan is a highly pure and soluble collagen that is produced via fermentation. It features a triple-helix structure to mimic the properties of natural collagen, making it suitable for use in medical, pharmaceutical, cell culture, tissue and other life science applications.
“Next-generation materials, like our collagen, inspire the creation of new and safer medical applications that previously might not have been feasible,” said Maximillian Yeh, Head of the Health Solutions product line at Evonik’s Health Care business. “We are excited to bring this nature-identical biomaterial to market at large scale.”
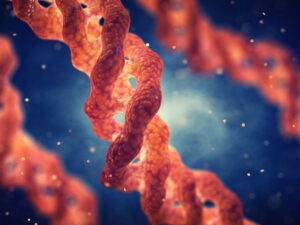
Evonik’s recombinant, non-animal-derived
collagen platform is now available at commercial scale. Vecollan is a highly pure and soluble collagen that is produced via
fermentation. Pictured is a collagen triple helix molecule. Photo courtesy of Evonik.
Vecollan is the latest addition to Evonik’s differentiating system solutions for the medical device industry, and is backed by application, development and manufacturing services from its medical device application centers in Birmingham, Alabama; Darmstadt, Germany and Shanghai, China. The fermentation-based production process was developed together with the Evonik innovation unit Creavis, and leverages the biotechnology platform of the company’s life sciences division Nutrition & Care. The division aims to grow its share of system solutions from 20% today to more than 50% by 2030.
“Our collagen – Vecollan– is highly tuneable and customizable to different product forms like hydrogels, sponges or inks to support our customer’s development projects,” said Andreas Karau, Global Head of Biomaterials at Evonik’s Health Care business line. “Whether for regenerative medicine, tissue engineering, aesthetic dermatology or dental applications, we are committed to finding the most suitable solution for our customers.”




