
The orthopedic device company Hip Innovation Technology (HIT) recently received FDA Investigational Device Exemption (IDE) approval to begin clinical studies to evaluate the effectiveness of its Reverse Hip Replacement System (Reverse HRS). BONEZONE spoke with HIT’s CEO George Diamantoni to learn more about the first of its kind hip implant.
Reverse HRS Development
Congratulations on your IDE. How did you arrive at the idea for reverse hip replacement?
Diamantoni: Thank you. We view receiving the IDE as a transformational event for our company and a significant step toward the future commercialization of the Reverse HRS. The Reverse HRS was invented by Dr. Zafer Termanini, an orthopedic surgeon, to maximize currently available total hip replacement system benefits while providing additional unique solutions to unmet clinical needs.
Can you walk me through how your implant system differs from typical hip replacement systems on the market?
Diamantoni: The Reverse HRS, as the name implies, is a reverse geometry hip prosthesis designed to improve stability throughout functional ranges of motion, reduce prosthesis impingement, and reduce the risk of dislocation. Dislocation continues to be one of the most common complications of conventional total hip systems and one of the most frequent indications for patients requiring reoperation.
The Reverse HRS consists of a cementless acetabular component that uniquely has a central taper into which the ball seats. Notably, the articulation surface is still a ball against a polyethylene liner. However, the system is reversed with the ball now associated with the acetabular component and the liner being associated with the femoral component. We believe this facilitates key product features, including improved stability, reduced impingement and reduced dislocation.
We cover the market closely and can’t recall hearing of a reverse hip. Has this been tried before?
Diamantoni: This novel approach has not been done before. HIT’s Reverse HRS possesses a patent-protected design that we believe represents a breakthrough technology that will significantly reduce common, yet challenging complications associated with total hip arthroplasty (THA).
The company has been internally focused on advancing all facets of development for our lead product. We have only recently initiated external product communications such as conference presentations and publications. Obviously, the news of the IDE has created broad awareness and heightened interest.
In the joint reconstructive hip market, there clearly exists a need for device improvement and innovation. Since its inception in the late 1960s, primary hip replacement has helped tens of millions of people overcome painful arthritis, recover from hip fractures and improve their quality of life. However, hip implants do not come without risk or complications.
The company’s Reverse HRS intellectual property portfolio contains over 180 issued and 110 pending U.S. or international patent applications. These U.S. patent applications and market-targeted international counterpart applications provide greater portfolio strength and key country protection.

The Reverse HRS consists of a cementless acetabular component that uniquely has a central taper into which the ball seats. The system is reversed, with the ball associated with the acetabular component and the liner being associated with the femoral component.
Commercialization Efforts
You received an FDA IDE to initiate a pivotal clinical study. What metrics are you observing? What is the size and duration of the study?
Diamantoni: It is worthwhile to note that we remain appreciative of FDA’s collaborative approach throughout this regulatory process and the resulting alignment on study design, patient size and endpoints. The clinical study objective is to evaluate the safety and effectiveness of the Reverse HRS in patients undergoing THA. Safety will be assessed by collecting device-related adverse events and patient quality of life metrics. Effectiveness will be evaluated using clinical, radiologic and patient-reported outcomes.
These outcomes are consistent with an ongoing radiostereometric analysis (RSA) clinical study in Canada, with an additional specific focus on acetabular and femoral component fixation. This study’s RSA results demonstrated excellent fixation to bone with minimum migration at 24 months for both the acetabular and femoral components.
Initial RSA results are predictive for long-term fixation, and our study data predicts the HIT Reverse HRS to be at a low risk for aseptic loosening and revision at 10 years. As a result, based on similar patient populations, we expect that the U.S. pivotal study will observe similar results to the ongoing Canadian clinical study.
HIT was founded in 2011. What are some of your accomplishments over the last 10 years? What do you expect from the next 10 years?
Diamantoni: We founded HIT to provide a product offering to address unmet existing patient needs, which remain after years of trial and error with a variety of product offerings. The three primary THA complications outside of infection are:
- Instability and dislocation
- Component positioning/placement
- Edge loading
In our first 10 years, we designed and developed our Reverse HRS to safely and effectively address these three complications. During this startup period, HIT invested heavily in our intellectual property portfolio along with extensive research and development studies that led to the RSA clinical study in Canada.
Extensive bench-level testing has been completed on the Reverse HRS, including standard and uniquely designed tests. These tests were designed to aggressively challenge a completely assembled Reverse HRS system. The testing categories included:
- Dynamic Fatigue: Durability testing conducted under active or simulated load
- Static: Stationary testing to verify structural design criteria, structural integrity and effects of limit loads
- Tribology: Wear testing of surfaces in relative motion
The Reverse HRS demonstrated excellent performance with all testing challenges, including tribology, durability and validation of structural design criteria, structural integrity and effects of limit loads.
Standard wear testing was completed at “optimal” acetabular cup positioning (45° inclination/20° anteversion). In addition, modified testing occurred with a series of malpositioned acetabular cups to simulate potential clinical scenarios, and in both models, average annual polyethylene wear was determined. The wear results were extremely positive and consistent regardless of acetabular cup placement, even in cups with extreme malposition.
These data further validate a primary product benefit that the Reverse HRS design has reduced the relationship between acetabular cup placement and component wear. We believe that subsets of patients such as those with spinopelvic movement disorders, aberrant variants of native femoral or acetabular version, or other factors that predispose to implant malpositioning may be ideal candidates for our Reverse HRS.
Assuming that the Reverse HRS performs in the pivotal study in a manner consistent with observed clinical performance to date, we believe its product profile will compare favorably versus existing products on the market. Obviously, this will create a wide variety of strategic pathways for the company. We plan to continue strengthening our internal infrastructure and human resources as we evaluate optimal commercial pathways. In parallel, we will assess value-creating options which may accelerate the trial, use and adoption of the Reverse HRS for the many patients who may benefit.
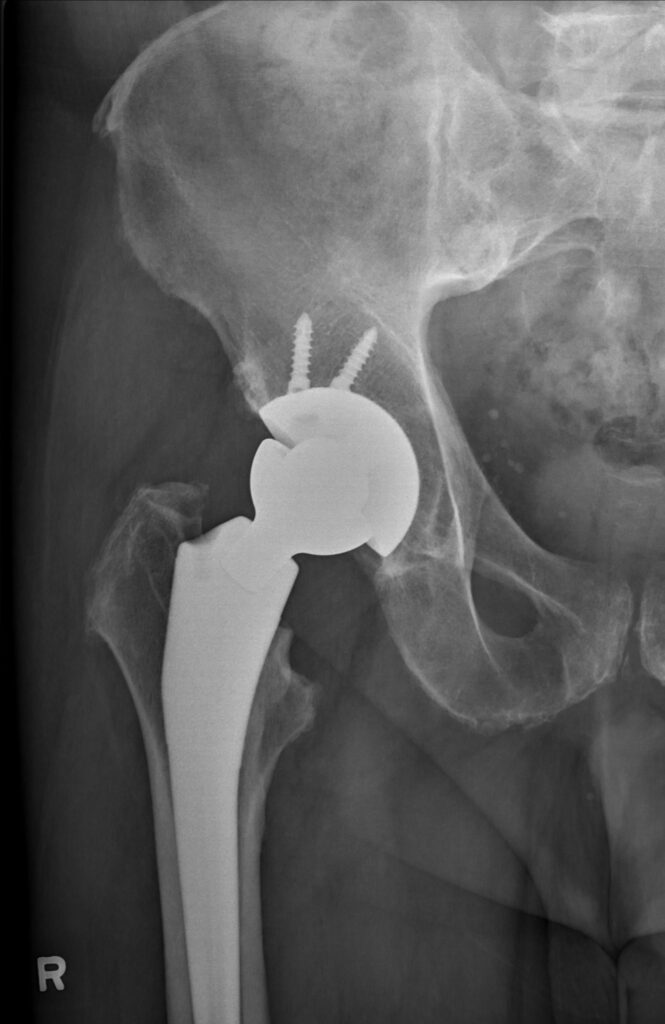
Results of a radiostereometric analysis clinical study in Canada demonstrated excellent fixation to bone with minimum migration at 24 months for both the acetabular and femoral components.
Hip Implant Market Trends
What market trends do you find to be the most encouraging and conversely, discouraging in today’s hip replacement market?
Generally, the life sciences industry is advancing technology forward at a faster rate than previously observed. Unfortunately, advances in the THA space have been incremental and unmet medical need remains. We believe our Reverse HRS exemplifies technology that will have a meaningful impact for total hip arthroplasty patients and the healthcare system through improved outcomes, reduced overall health care costs and improvement in long-term patient quality of life.
Other market trends of note include a consistent year-over-year increase in the number of THAs completed. As the population ages and the Baby Boomer generation moves into the demographic requiring THA, this trend will continue as the need for hip arthroplasty will significantly increase globally. This trend is further enhanced by individuals aging with a greater propensity for weight-related issues driving incidence rates of osteoarthritis higher in the general population resulting in more THAs.
Historically, access to THA in global emerging markets has been limited. Fortunately, there appears to be a trend for increased access to THA, which would benefit patients and further increase global demand.
PM
Patrick McGuire is a BONEZONE Contributor.




