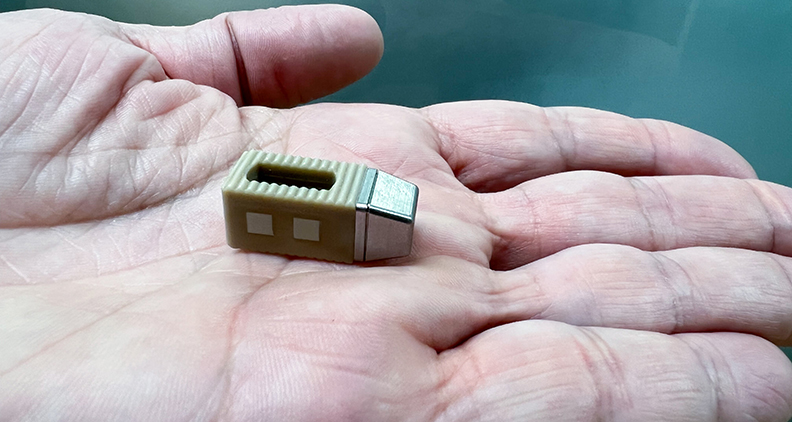
Startup Intelligent Implants received an FDA Breakthrough Device Designation new funding and a new CEO in recent months. The company’s SmartFuse technology is a wirelessly enabled orthopedic platform designed to remotely stimulate, control and monitor bone growth.
We spoke with Benjamin A. Hertzog, Ph.D., days after taking the helm at the company. We touched on various topics, from SmartFuse to the future of orthopedic technology and the pathway from engineer to executive.
What attracted you to Intelligent Implants?
Dr. Hertzog: I am a serial medical device entrepreneur, and frankly, I see Intelligent Implants as the future of medical devices. It’s what we have all been talking about in this industry for quite some time now: a smart, connected implant that provides therapeutic benefit AND remote data to support real-time clinical decision making. I believe that smart implants will have the ability to benefit patients well beyond what traditional medical devices are capable of.
Let’s talk about technology a bit. Can you describe Integrity Implants’ SmartFuse for me?
Dr. Hertzog: SmartFuse is a wirelessly enabled, active implant technology platform that uses an array of electrodes to stimulate, control and monitor bone growth. The system comprises an implant that delivers local electrical stimulation to accelerate bone growth, an external “wearable” to wirelessly power and communicate with the implant, and a cloud-based physician portal and patient app.


Integrity Implants is developing a wirelessly enabled, active implant technology platform that uses an array of electrodes to stimulate, control and monitor bone growth. Its first indication is spinal fusion.
How does it work? What sets it apart from other technology on the market?
Dr. Hertzog: In one respect, this is the local drug delivery version of electrical bone growth stimulators. We can precisely control the stimulation by doing the stimulation locally and with an array of electrodes. These same electrodes are then used to measure the new bone growth, and again, the electrode array enables us to detect the new bone and spatially resolve where the new bone is growing.
In a way, we have a technology with the ability to 3D-print new bone in situ, directly where you need it. Plus, we can monitor that growth and communicate that progress to the clinician in real-time through our SmartFuse Cloud physician portal. This capability just doesn’t exist today.
I understand that your first indication is lumbar spinal fusion. Why start with the spine? What other indications do you see in the future?
Dr. Hertzog: We see an obvious unmet need in spinal fusion. Despite all of the progress, the new technologies, and all the intelligent people focused on this space, there is still an unacceptably high rate of non-unions in the lumbar spine. There is a lot more we can do to improve outcomes for patients.
From a technology perspective, intervertebral cages are small. If we can miniaturize our technology to fit into a TLIF cage, for example, we will have a technology that we can incorporate into almost any orthopedic implant. We envision a pipeline of products across orthopedics that incorporate the SmartFuse technology: intramedullary rods, hip and knee implants, etc.


SmartFuse comprises an implant that delivers local electrical stimulation to accelerate bone growth, an external “wearable” to wirelessly power and communicate with the implant, and a cloud-based physician portal and patient app.
In October, the company announced new funding and an FDA Breakthrough Device Designation. What does your commercialization timeline look like?
Dr. Hertzog: We all know this takes a long time. The FDA Breakthrough Designation was a big win for us. For what it’s worth, the current FDA is more pro-innovation than possibly any time in history, and our personal interaction has been with an FDA team that is engaged and highly collaborative. All of that should help with the timeline.
As a company, our sole focus right now is on getting the SmartFuse system into the clinic for a first-in-human study for spinal fusion. That being said, we are trying to do everything we can from a product development perspective to accelerate the timeline and make the path from bench to clinic to market as fast as possible.
We see more technology – be it robotics or other digital and wireless technologies – being incorporated in orthopedics. What market forces are making the adoption of technology more attractive in orthopedics today?
Dr. Hertzog: That’s a great question. I think we have all seen how impactful data and real-time access to that data has been in our day-to-day lives. We have also seen its impact in other industries, so it is easy to connect the dots and say that it should produce significant benefits in medical devices.
I think a major challenge has been the business model. Data in and of itself is diagnostic in nature, and we have struggled as an industry to quantify the value of that data and figure out who pays for that value. For example, we have been talking about smart orthopedic implants for some time now, but that discussion has focused chiefly on adding sensors to existing implants.
As engineers and clinicians, we can imagine all kinds of interesting data we would like to see (e.g., quantification of motion, force, strain, etc.). We also agree that we will probably learn something from that data, but much of that value is created and captured in the future. So, who is going to pay for that now? And how much?
A significant differentiator for Intelligent Implants’ SmartFuse system is that it provides a therapeutic benefit (i.e., accelerated bone growth). That benefit is immediate, and the business model is well understood. In a sense, the data (i.e., the remote bone growth monitoring) gets to ride along for free from a technology perspective.
By combining the therapeutic benefit with the monitoring/data capability, I think this presents us with an opportunity to rethink the historical business models. That is very exciting to me.
Our readership is primarily orthopedic R&D decision-makers. You started with an engineering background. How do you think that background has influenced your decisions as an executive and entrepreneur?
Dr. Hertzog: The technology has to be good. It has to work, and it has to work reliably. It has to be safe. It has to provide a benefit to the patient. If the technology isn’t sound, you have nothing.
However, good technology is not enough. The commercialization risks are many, and unfortunately, none of these great ideas help patients unless they are commercialized.
I pride myself on being able to operate at that intersection of technology and commercialization. Product development must focus on the technology, but that process must also be compatible with the realities of market development, regulatory, clinical and finance. That breadth of experience is critical to being successful in this field.
You were most recently the Entrepreneur in Residence with Johnson & Johnson’s Center for Device Innovation at the Texas Medical Center. What were some of the greatest lessons you learned during that time?
Dr. Hertzog: J&J’s Center for Device Innovation at The Texas Medical Center (CDI@TMC) is an exceptionally unique model. It’s designed from the ground up as a place where medical device innovations can move quickly from “crazy idea” to proof of concept with a fleshed-out business plan. It was a great privilege to get to spend my last couple of years there working with some incredible innovators.
It is no secret that large companies struggle with innovation yield. This is not unique to medical device companies, but rather is a systemic problem faced by all industries. The skills, behaviors and processes that typically enable a big company to succeed are often 180 degrees out of phase with what it takes to be innovative, take risks and move fast.
I give my good friend, Dr. Billy Cohn (surgeon, innovator, and Executive Director of CDI), and Johnson & Johnson huge credit for being willing to rethink the business model for medical device innovation within a large company. They created a place where that can happen quickly with all of the skills, talent and resources necessary…but without the typical big-company constraints and hurdles.
So, in broad strokes, my answer comes back to my earlier comments about rethinking business models. Seeing a large company like J&J be successful at the scale of CDI made me appreciate the fact that all of us entrepreneurs have no excuses. We must continue to push the boundaries and always be willing to rethink the business model to stay at the leading edge of the innovation curve.
PM
Patrick McGuire is a BONEZONE Contributor.




