
This month’s recap shares orthopedic companies that received their first 510(k) clearances within 2022, featuring applications for spine, trauma and robotics/digital. Notably, the group includes the first clearance for the company named ZimVie, Zimmer Biomet’s spinoff for spine and dental products.
Spine
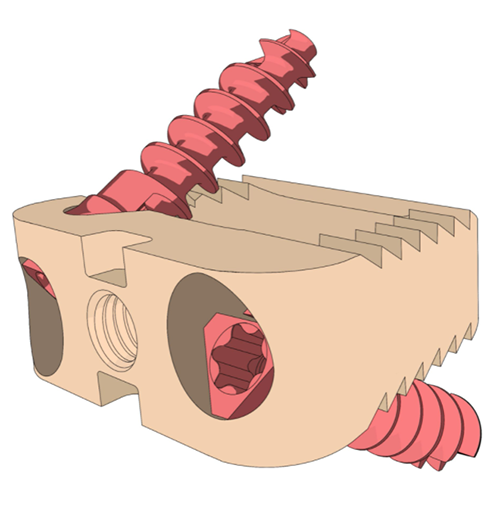
SpineUp Romero Cervical Cage
SpineUp | Romero Cervical Cage, K212358
- Submitted July 2021, granted January 2022
- Primary predicate: Medtronic DIVERGENCE Anterior Cervical Fusion System, K141599
- About the product: Lordotic and anatomic self-anchored anterior cervical interbody device comprising a PEEK HA or PEEK OPTIMA interbody cage with tantalum radiographic markers and two titanium fixation screws for cages with screw holes
- About the company: Founded in 2018, based in Miami, Florida. Partnered with Safe Orthopedics for product co-development and distribution in the U.S. and a few other strategic territories. SpineUp team has over 25 years of collective experience in the spinal industry, including time at Spineway, as well as Small Bone Innovations.
Trauma
Fusion Innovations | SternaFuse Fixation System, K202914
- Submitted September 2020, granted January 2022
- Primary predicate: Biomet Microfixation Sternal Closure System, K111908
- About the product: Indicated for use in the stabilization and fixation of fractures of the anterior chest wall, including sternal fixation following sternotomy, sternal fractures and sternal reconstructive surgical procedures, to promote fusion
- About the company: Founded in 2012, located in Maitland, Florida. The first company to develop and introduce the idea of using a “fusion strip” to reconstruct sternal defects.
NEOS Surgery | Stern Fix Sternal Stabilization System, K211613
- Submitted May 2021, granted January 2022
- Primary predicate: Synthes Sternal ZipFix System, K110789
- About the product: Intended for closure and stabilization of the sternum following sternotomy, through the intercostal spaces, in order to promote its fusion
- About the company: Established in 2003, based in Cerdanyola del Valles, Barcelona. NEOS Surgery has raised a total of $8.4 million in funding over six rounds. In 2020, the company received €1.9 million (~$2.2 million) from the European Commission to support clinical validation of the DISC care device, which has been designed based on NEOS Surgery’s neurosurgery expertise to respond to an unmet medical need, lumbar disc herniation.
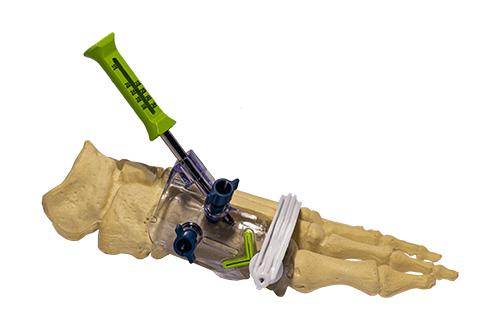
RELJA Innovations MIS Precision Chevron Bunion System
RELJA Innovations | MIS Precision Chevron Bunion System, K211628
- Submitted May 2021, granted January 2022
- Primary predicate: Stryker Fixos Screw, K070039
- About the product: Indicated for fixing and stabilizing the elective osteotomies of the mid-foot bones and the metatarsal and phalanges of the foot only. Comprises a single, sterile-packaged SKU that contains both implants and instruments needed for the procedure.
- About the company: Established in 2019, located in Brookfield, Wisconsin. CEO is a practicing physician; COO brings experience from roles at BioMedical Enterprises and DePuy Synthes.
Robotics/Digital
ZimVie (Zimmer Biomet Spine) | Vital Navigation System, K213720
Submitted November 2021, granted January 2022
- Primary predicate: Zimmer Biomet Spine, Vital Navigation System, K191722
- About the product: Vital Navigation comprises nonsterile, reusable instruments intended for use with the Medtronic Synergy Experience StealthStation System to locate anatomical structures in open or minimally invasive procedures, to prepare and place Vital and Vitality screws. This imaging technology provides visualization for complex and MIS procedures and confirms the accuracy of advanced surgical procedures. Use of these navigation systems provides the surgeon access to real-time, multi-plane 3D and 2D images, lending confirmation of hardware placement. Through the introduction of new styluses in this submission, the previously cleared Vital Navigation Taps and Reduction Driver can be used with the existing Vital MIS PAT Handle to allow the user to navigate while using the PAT or PASIT assembly. Company’s first product clearance under the ZimVie name.
- About the company: In early 2021, Zimmer Biomet announced its intent to spin off its spine and dental divisions into a new publicly-traded company called ZimVie. The spin-off will complete on March 1, 2022.
JAV
Julie A. Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.




