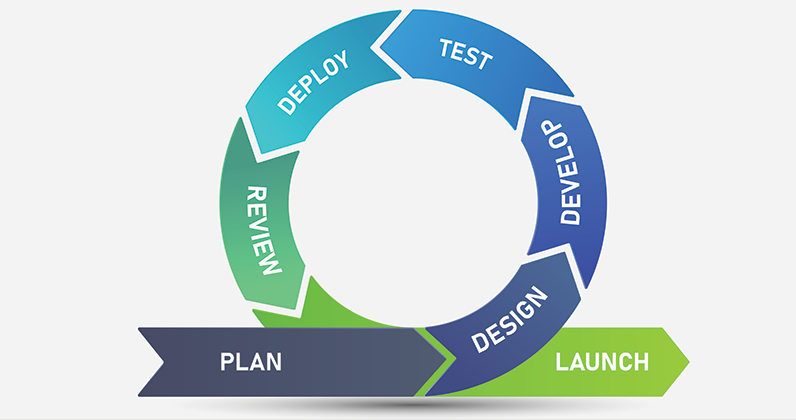
Orthopedic device development can be a lengthy and expensive process. Failure to properly navigate the development, quality and regulatory requirements can lead to gross timeline and budget expansion and potentially project failure. This webinar guides OEMs, clinicians and entrepreneurs on quickly getting their ideas to market with an integrated development process.
Outsourcing product development needs allows you to move on ideas stuck in your pipeline while focusing on your core products and business. Additionally, leveraging a vertically integrated supplier for product development can make for a smoother design transfer process.
Lincotek Medical has assembled a team of professionals that provides an integrated experience for developing orthopedic implants and instruments. It includes robust QMS, regulatory services, dedicated prototyping, testing and an onsite cadaver lab, design transfer and full-scale manufacturing. Lincotek’s combined product development and regulatory experience has led the company to submit nearly 200 regulatory submissions in the U.S. and CE markets.
This webinar provides an understanding of the advantages of an integrated experience for the development of orthopedic instruments and implants.