
This year, our survey of orthopedic professionals revealed that robotic and other digital tools were among technologies that respondents felt would disrupt orthopedics the most in coming years. I always love the opportunity to share lesser-known companies in this column—here are five that gained FDA 510(k) clearance this year for products in this segment of the market.
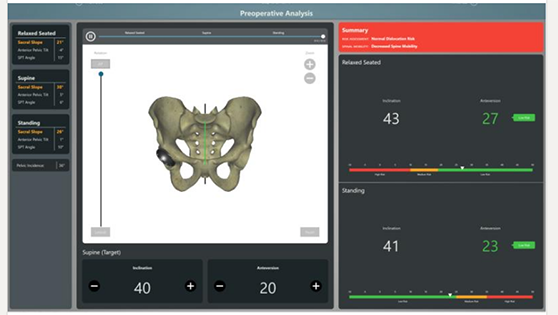
Cuptimize Software for Hip Applications
Cuptimize | Cuptimize Image-processing Software, K203651; submitted December 2020, granted February 2021
- Predicate device: JointPoint, K160284
- About the product: Image-processing software that provides a preoperative and intraoperative solution for hip replacement, to determine acetabular cup placement. It relies exclusively on x-ray, and requires no CT scan for the preoperative analysis module, and integrates directly with intraoperative navigation solutions. This is the company’s first 510(k) clearance.
- About the company: Founded in 2020, based in Belleair Bluffs, Florida. Leadership brings experience at JointPoint (which was acquired by DePuy Synthes).
Genesis Software Innovations | PreView Shoulder, K210556; submitted February 2021, granted April 2021
- Predicate device: Peek Health PeekMed pre-operative planning software, K182464
- About the product: 3D shoulder replacement planning software based on CT imaging studies. PreView shows a representation of the patient’s shoulder anatomy as a 3D model, allowing for digital placement of the implant and optimization of implant size, location and orientation. This clearance is the first orthopedic solution under FDA’s new QIH classification for software solutions based on Artificial Intelligence, and is also the company’s first 510(k) clearance.
- About the company: Established in 2019, based in Holland, Michigan. Portfolio includes tools to address inventory and sterilization for hospitals and surgery centers, for data collection and patient satisfaction and patient optimization software to assist in surgery preparation and recovery. Has received investments from Genesis Innovation Group and cultivate(MD).

Micromate Robotic System
Interventional Systems/iSYS Medizintechnik | Micromate, K203720; submitted December 2020, granted June 2021
- Predicate device: iSYS Medizintechnik iSYS1 Robotic Positioning Unit, K131433
- About the product: Reportedly the world’s smallest robot for percutaneous procedures. It can perform almost every non-invasive image-guided procedure, and is available in the U.S. spine fusion landscape through partner company, Fusion Robotics. The system is table-mounted for interventional procedures with a universal instrument guidance solution that allows physicians to continue using their preferred surgical instruments. Targeting is performed under live-imaging using any Cone-Beam Computerized Tomography, Fluoro CT or a Fluoroscope.
- About the company: Founded in 2010, headquartered in Tyrol, Austria. Launching U.S. operations via centers of excellence for robotic-guided interventional procedures and establishing commercial partnerships with third parties that seek a robotic offering for their customers. CEO is also CTO for partner company, Fusion Robotics.
Naviswiss | Naviplan CT Planning Software For Total Hip Replacement, K211429; submitted May 2021, granted October 2021
- About the product: A digital CT-based pre-operative planning application that assists the surgeon in the optimal positioning of the joint implants, automatic 3D segmentation and advanced image processing. Naviplan outputs the pre-operative plan into the Naviswiss navigation platform for accurate surgical execution.
- About the company: Established in 2007 and headquartered in Brugg, Switzerland. Has raised a total of CHF 9.5 million (~USD $10.5 million) in funding over three rounds. CEO is co-CEO at development company Medivation, and brings experience in computer-assisted surgery at Smith & Nephew. Others in leadership have held positions at Blue Belt Technologies, Intellijoint Surgical, Mazor Robotics, Medtronic, Stryker Navigation and Sulzer Orthopaedics.
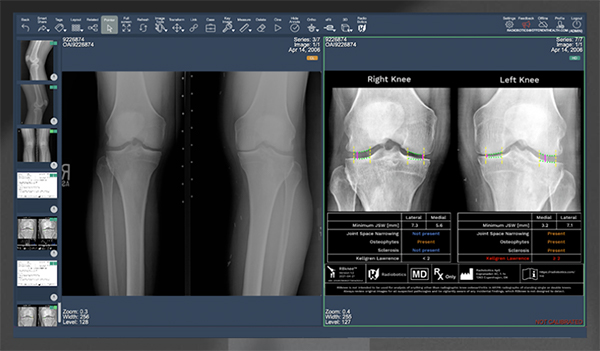
Radiobotics RBknee AI-based Analysis Tool
Radiobotics | RBknee, K203696; submitted December 2020, granted August 2021
- Predicate device: IB Lab KOALA image-processing software, K192109
- About the product: An AI-based analysis tool aimed at improving the efficiency and accuracy of radiographic knee osteoarthritis (OA) diagnosis. Intended for use in an orthopedic or a radiological setting, where the x-ray is read in order to determine the diagnosis and treatment of OA. RBknee can identify presence or absence of osteophytes, subchondral sclerosis, and joint space narrowing using proprietary machine learning algorithms. Furthermore, RBknee can accurately measure the joint space width in both compartments of the knee. RBknee is also CE Marked.
- About the company: Founded in 2017, located in Copenhagen, Denmark. Raised $4 million in funding over five rounds. Has also developed RBhip to automatically detect findings relevant for radiographic hip osteoarthritis, together with automatic measurement of common angles. Leadership brings experience from Embody Orthopaedic. Earlier this year, Radiobotics unveiled its first subsidiary, Radiobotics Inc. in the U.S.
JAV
Julie A. Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.




