
Several recent product launches caught our attention due to the application or the technology itself. I dove into five of the latest products from companies like Anika Therapeutics, ExplORer Surgical and Smith+Nephew.
Of note, ExplORer Surgical, Intelivation Technologies and Nanova Biomaterials are lesser-known in the industry, established in 2015, 2020 and 2013, respectively.
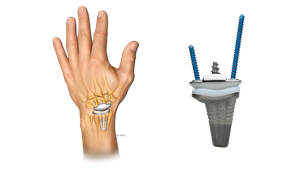
Anika WristMotion Total Wrist
Limited Launch, First Case with Anika Therapeutics’ WristMotion Total Wrist Arthroplasty
WristMotion TWA, cleared by FDA in 2020, is a modular preservation system that replaces both the radial and carpal sides of the joint. The system incorporates Anika’s fixation technology in the central carpal taper post design, also used in the WristMotion Hemiarthroplasty system. This system design addresses the concern of carpal loosening as it relates to existing systems.
WristMotion System also employs a dual curvature design feature in the carpal implant combined with the dorsal flange, supporting greater range of extension due to the increased implant surface area. Preclinical robotic testing has demonstrated that the combination of these features provides exceptional range of motion and rotational.
Full U.S. launch will occur in late September.
Explorer Surgical Robotic Solution
ExplORer Surgical Expands Offerings into Robotic Surgery
ExplORer Live, reportedly the first cloud-based digital platform customized for medical device companies to share best practices live with surgical teams, is growing its portfolio into robotic surgical applications, including orthopedic procedures.
Medical robotics companies can now leverage ExplORer Live, a digital platform featuring a first-of-its-kind procedural playbook, video capabilities and robust data collection to provide support and insight into workflow and performance.
Easing the learning curve surrounding robotic surgery enables and supports surgeon proficiency, clinical team satisfaction and reduced O.R. inefficiencies, all of which encourage medical device adoption and increase hospital revenues for a return on investment.
With ExplORer Live, surgical teams and robotics companies have access to all images, PDFs and videos about the system; surgical techniques and a step-by-step workflow for each team member in the O.R. The digital playbook is customized to the clinical team’s role, procedure type and surgeon preferences. Surgeons can broadcast in real-time to provide clinical education, remote collaboration or demonstrations to potential users. The platform can capture robust video and procedural data used by a surgeon or in partnership with a robotics company for performance tracking, identifying opportunities to reduce variability, postmarket feedback and analysis and future product development.
Intelivation Technologies Debuts the TruTrack Memory Staple
The TruTrack system comprises a shape memory alloy staple that provides dynamic compression in multiple applications. A Nitinol staple provides constant compression to help bone healing, while an S-shaped bridge offers even compression across the fusion site.
TruTrack features novel instrumentation designed for reliable, consistent insertion techniques during a variety of intraoperative conditions.
Compared to traditional methods that can lead to drill hole obscuration, TruTrack lends itself toward less soft tissue disruption, resulting in improved surgical outcomes for patients. The implant is low profile and can be applicable to a variety of degenerative conditions and deformities of the foot.

NanovaBio FiberFix Screw
New FiberFIX Suture Anchors, Interference Screws from Nanova Biomaterials
Nanova Biomaterials’ FiberFIX suture anchors and interference screws have demonstrated enhanced resistance to breakage/stripping during insertion and increased pull-out strength after-insertion, which reduces the failure rates during orthopedic procedures. Real-time degradation tests have also indicated adequate mechanical stability for three months and no sign of self-catalytic degradation over two years of observation, which brings significant benefits in soft tissue fixation in sports medicine.
FiberFIX represents a new generation of bioresorbable materials developed using nanotechnology: biocomposites enhanced by specially modified single crystalline hydroxyapatite nanofibers. Nanofibers are about 100 nanometers in diameter and tens of micrometers in length with a strength close to its theoretical strength, several times stronger than stainless steel.
Just like re-bar in concrete, specially modified nanofiber may effectively strengthen the mechanical properties of the composites. Furthermore, with over 100 times greater surface area than traditional calcium phosphate particles, the modified nanofibers improve the acid buffering ability of FiberFIX devices, reduce acidic degradation of the composites and minimize the body’s inflammatory response.

Smith+Nephew FAST-FIX FLEX
Smith+Nephew FAST-FIX FLEX Meniscal Repair System
FAST-FIX FLEX Meniscal Repair System offers a surgeon-guided, bendable needle and shaft providing access to all zones of the meniscus.
The new system uses an all-inside approach which may eliminate the need for further incisions, reduce the risk of neurovascular injury and provide procedural efficiency to support faster operating times. The added ability to bend both the needle and shaft enables access to the mid-body and anterior zones, inaccessible by previous FAST-FIX devices.
FAST-FIX FLEX offers a 25% reduction in needle insertion area as well as a repair that is more than 20% stronger than the previous generation FAST-FIX 360 System.
JAV
Julie A. Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.




