Readers often ask for help identifying new companies. With this feature, we seek to share startups and companies that we’ve recently come across. These entities may have received funding, a notable FDA 510(k) clearance, made acquisitions or entered into partnerships.
In this round, we highlight companies with products for robotics/digital, spine and orthobiologic applications.
Activ Surgical Gains FDA Clearance for ActivSight Intraoperative Imaging Module for Enhanced Surgical Visualization
In April 2021, Activ was granted FDA 510(k) clearance to market ActivSight, a hardware-agnostic imaging module designed to provide surgeons a real-time intraoperative visual data and imaging option not currently available with existing technologies. Initial launch is slated for 2H21.
ActivSight will power the company’s next product, ActivInsights, which will be part of the ActivEdge software platform, employing artificial intelligence (AI) and machine learning (ML).
The first ActivInsight that will be made available is perfusion insights, and will offer the ability to see blood flow and perfusion in real time, without the use of traditional dyes.
The company was founded in 2017 and has raised a total of $40.4 million in funding to date over four rounds; the latest comprised $15 million in 2020. Leadership experience includes roles at Baxano, Boston Scientific, DePuy Spine, NuVasive, Olympus, Smith+Nephew, Stryker and Zimmer Biomet.


Activ Surgical ActivSight
Amplicore Closes Seed Funding Round
Amplicore closed its series seed round of funding at $4 million. Proceeds will allow the company to complete preclinical development of its two leading products, AM3101 and AM1101 injectables, and position the company for the next round of financing. This builds upon a first seed round which raised $2.8 million and a $2 million government grant.
Amplicore is pursuing a regenerative approach to treat joint osteoarthritis, cartilage damage, degenerative disc disease and acute meniscus tear. AM1101 is in development as a treatment for osteoarthritis designed to reduce pain, inhibit cartilage degradation and promote tissue regeneration of aberrant cartilage.
The company is also leveraging a study on the healing of acute meniscal tear after surgical repair, supported by the grant from the U.S. Department of Defense. AM3101 technology has demonstrated promising preclinical results as a potential minimally invasive and regenerative approach to managing soft tissue injury.
Amplicore was established in 2019. The prior venture of the President and CEO was co-founder of Tissue Regeneration Systems, whose suite of technologies was acquired by DePuy Synthes and licensed to Medtronic.
CornerLoc Developing the CornerLoc SI Joint Fusion System
The CornerLoc™ sacroiliac (SI) joint stabilization procedure was designed to support stabilization and fusion potential, with minimal surgical invasiveness. After preparation of the SI joint, two CornerLoc grafts are placed orthogonally within the SI joint, creating immediate joint stability and an optimized environment for fusion.
CornerLoc cortical graft plus a DBM sponge combine to reduce pain and instability within the sacroiliac joint.
The system is the subject of a randomized, multicenter, open-label U.S. clinical trial comparing CornerLoc to steroid injections to treat SI joint dysfunction.
The company was founded in 2017.
Cuptimize Gains 510(k) for Cup Placement Software
Cuptimize received FDA 510(k) clearance for spinopelvic tilt analysis software that provides a preoperative and intraoperative solution for hip replacement. The software is now available in the U.S.
The software relies exclusively on x-ray, and requires no CT scan for the preoperative analysis module.
Cuptimize allows hip surgeons to understand when the patient has a problem, determine cup placement to mitigate risk and have clear indications of when to use dual mobility implants. The system also integrates directly with intraoperative navigation solutions.
Cuptimize was established in 2020. The company president is the former leader of JointPoint, a provider of non-invasive navigation for hip surgery. The digital platform was acquired by DePuy Synthes in 2019.
Kolosis BIO and MTF Biologics Launch Kore Fiber Allograft
Kolosis BIO and MTF Biologics released Kore Fiber™, an exclusively processed demineralized cortical fiber allograft for Kolosis.
Kore Fiber is available in four different sizes of moldable fiber and three sizes of strips. Head-to-head testing has demonstrated that Kore Fiber has higher osteoinductivity than commercially available grafts from Medtronic, SeaSpine and Zimmer Biomet.
Kolosis exclusively partners with MTF to deliver bone graft technologies in the orthopedic space. The company’s initial tissue offering, Prime HD, is exclusively represented by Kolosis through its network of independent distributor partners. Prime HD is a pre-hydrated cortical matrix treated with MTF’s proprietary aseptic processing methods, and features excellent handling characteristics and convenient delivery, making the graft ideal for a variety of challenging clinical applications, especially where cost is a primary consideration.
Kore is an aseptically-processed, 100% cortical bone matrix with ease of handling characteristics. Extensive pre-launch testing has shown Kore’s dynamic spectrum of growth factors that are essential for promoting and accelerating bone healing.
Kolosis exclusively partners with MTF to deliver bone graft technologies in the orthopedic space. The company was founded in 2020. Leadership brings experience from roles at Cook Medical, Lanx, Medtronic, NuVasive, Orthofix, Prosidyan, SI-BONE and Smith+Nephew.
Signature Biologics Initiating Signature Cord Prime Phase I Study for Knee Osteoarthritis
Signature Biologics received FDA approval of an Investigational New Drug Application (IND) to proceed with the study of Signature Cord Prime™ in patients with symptomatic knee osteoarthritis (OA).
Signature Cord Prime cryopreserved, human umbilical cord tissue allograft comprises micronized umbilical cord tissue. The product contains naturally occurring cellular growth factor, pro angiogenesis, anti-inflammatory cytokines and extracellular components.
FDA granted IND approval based on multicenter, retrospective clinical evidence from 135 knees treated with Signature Cord™ that demonstrated both safety and statistically significant reduction in pain and improvement in function.
This represents a push for regenerative medicine as the first hUC [human umbilical cord] product of its kind to receive approval to be studied in osteoarthritis of the knee.
Signature Biologics was founded in 2018.
Sparta Biopharma Launches Allograft for Rotator Cuff Repair, Gains Breakthrough Device Designation for SBM-01 Biomimetic Implant
Sparta Biopharma launched BioEnthesis™, an allograft that possesses both mineralized and demineralized layers intended to support healing and repair within the rotator cuff.
BioEnthesis biphasic cancellous bone allograft has an intact porous collagenous matrix for soft tissue ingrowth, and a highly calcified layer to promote osseointegration. It serves as a scaffold to repair the bone of the enthesis and provides a foundation to facilitate tendon incorporation.
The company plans to focus on alternatives to knee replacements, biological options for rotator cuff surgery and a natural alternative to delay a hip replacement.
Sparta Biomedical was also granted FDA’s Breakthrough Device Designation for the SBM-01 Biomimetic Implant. SBM-01 is intended to replace damaged knee cartilage in patients having single or multiple chondral or osteochondral defects in the knee.
SBM-01 mimics the properties of native cartilage and provides a smooth articulating surface, offering support to surrounding cartilage while stabilizing subchondral bone, thereby limiting further exacerbation of the disease.
The company was formed in 2017.
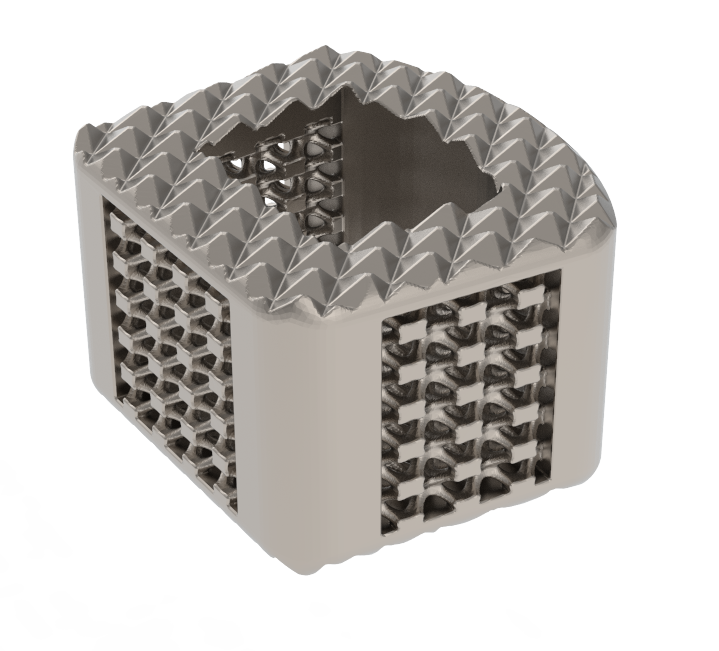

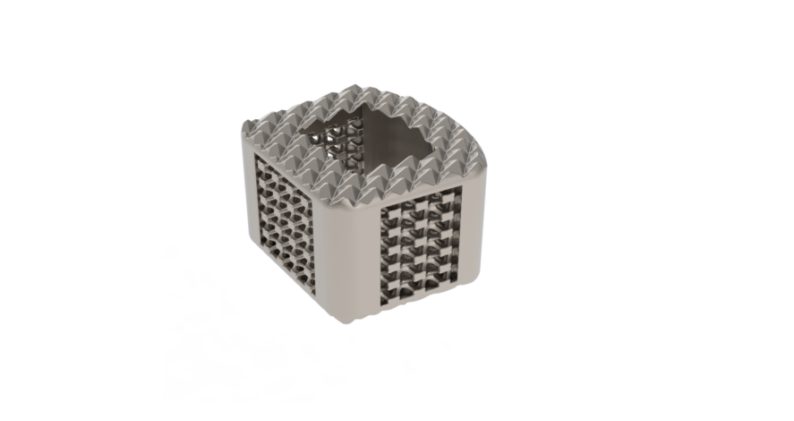
ZSFab Cervical Cage
ZSFab Received FDA 510(k) Clearance for the ZSFab Cervical Interbody System
ZSFab was granted FDA 510(k) clearance to market its first 3D-printed cervical interbody system, making ZSFab the first Chinese company to receive FDA 510(k) clearance for 3D-printed orthopedic implants.
The system features an interconnected porous structure with sub-micron rough surfaces, engineered for bony integration throughout the endplates. The porous structures and central graft window lower the stiffness to help reduce the stress shielding effect and minimize subsidence, accordingly. In addition, the optimized design makes the implants with high fatigue strength, exceeding more than 95% of similar cervical devices cleared by FDA.
ZSFab is dedicated to producing high performance, patient-matched as well as customized medical implants, surgical guides and other additively manufactured end-use products, and is developing solutions by integrating proprietary algorithms, advanced modeling and optimization methods, and 3D printing technologies, for healthcare and related areas.
Established in 2017; headquartered in Cambridge, Massachusetts with a facility in Beijing, China, ZSFab has also developed 3D-printed lumbar cages and a vertebral body replacement, as well as hip replacement components (not available in the U.S.). The company also offers surgical planning and digital modeling services.
JAV
Julie A. Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.




